AI supports breakthrough ALS research
New research from the University of St Andrews and the University of Copenhagen has harnessed the power of AI to generate new insight into the progression of ALS.
ALS (Amyotrophic Lateral Sclerosis) is a fatal disorder in which motor neurons – the cells which control movement – progressively die. There is no cure for the disease, and life expectancy after diagnosis is generally between 2 and 5 years.
The new study, published in Science Advances, responds to the urgent need for new knowledge about ALS in the search for effective treatment and a cure.
The research, led by researchers from the School of Psychology and Neuroscience at the University of St Andrews, in collaboration with the Department of Neuroscience, University of Copenhagen, shows that specific cell circuits which control movement are affected early in the disease, while others are affected later during disease progression.
“By using techniques that allow to simultaneously study multiple cell types in spinal cord tissue, combined with a novel AI-based analysis method, we identified the specific networks of cells affected early in disease before motor neurons die”, explains lead researcher Dr Ilary Allodi, lecturer in Systems Neuroscience at the School of Psychology and Neuroscience. “These are subgroups of inhibitory interneurons – a type of cell found in the spinal cord known to activate motor neurons.”
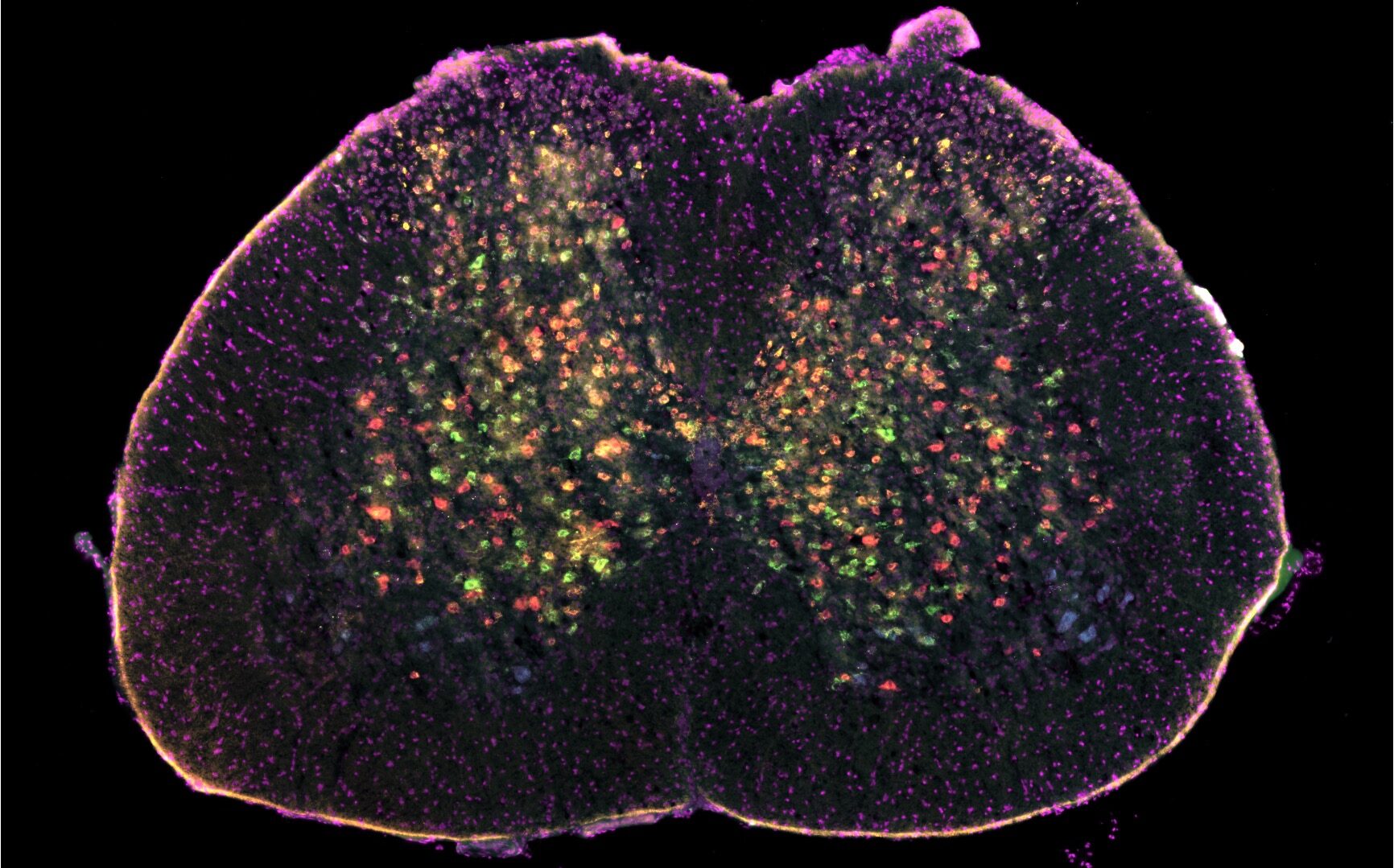
“In healthy individuals, these cell circuits are required to perform movements as walking and running”, adds Ole Kiehn, Professor in Integrative Neuroscience and co-corresponding author of the study. “There are specific cells, called inhibitory or excitatory interneurons, which control different aspects of movement by activating motor neurons. We found that some of these cells are affected at different stages of ALS, with the inhibitory interneurons being affected early on and the excitatory ones being affected later during disease progression”.
Researchers developed an AI-based method to facilitate data quantification. “The work uses cutting-edge methodology to identify the cell types contributing to disease”, explains Dr Roser Montañana-Rosell, first author of the study. “These inhibitory and excitatory cells are highly heterogeneous and intermingled within the spinal cord and often difficult to investigate simultaneously. The computational approach we developed allows to overcome these limitations, while shedding light on potential new targets for treatment.” This method is accessible online, with the hope that it will facilitate the completion of other similar studies.
The research was performed in partnership with 10X-Genomics and ACD Bio (Biotechne), two industrial organisations with a leading role in the field of transcriptomics, the technique which enables the identification of cells within the spinal cord.
“We used techniques that allow us to visualise and quantify multiple genes at the same time with single cell resolution in the spinal cord of the ALS mouse model”, says Dr Allodi.
“Each cell type can be identified by a specific set of genes, but these genes need to be visualised simultaneously. By using these transcriptomic techniques, we were able to differentiate between inhibitory and excitatory populations and among their subpopulations. This allowed us to investigate their fate during different stages of disease progression.”
Category Research