Breaking bonds
A newly discovered form of bacterial adhesion could be an answer to the rising crisis of antibiotic resistance.
Scientists at the University of St Andrews have discovered a special type of bacterial binding, and their developing research suggests it may be possible to prevent some of the world’s most dangerous bacteria from latching onto human cells.

One of the greatest threats to global health is the emergence of bacterial resistance to antibiotics. At the moment, the most widely used and often the only way to cure or prevent bacterial infection is to destroy the bacteria through antibiotics. However, over time, bacteria can develop resistance to antibiotics, and this resistance can have devastating results, up to the point where there are no treatment options for infections that previously could be controlled by antibiotics.
We’re in an arms race with bacteria.
Dr Schwarz-Linek

Infection occurs only once bacteria are able to attach to human cells, allowing them to invade their host, multiply and cause disease. In order to develop better treatments for bacterial infections, a team of biomolecular scientists from St Andrews wanted to conduct further research into understanding how bacteria are able to gain a foothold in the human body in the first place.

The team, led by Dr Uli Schwarz-Linek, have discovered a new type of bonding mechanism found in certain bacteria responsible for some of the world’s most prevalent and destructive diseases, including strep throat and pneumonia. The team was funded by the Medical Research Council and works in collaboration with Professor Mark Banfield from the John Innes Centre Norwich and Professor Manfred Rohde from the German Helmholtz Centre for Infection Research.

News of this discovery spread quickly, picked up by The Times and The National, and highlighted in an article by Professor Ted Baker, one of the world’s leading structural biologists. You can listen to an interview by Dr Chris Smith from the University of Cambridge with Dr Schwarz-Linek below.
“Chemical Harpoons: bacterial anchors” interview with Dr Uli Schwarz-Linek by Dr Chris Smith, University of Cambridge, The Naked Scientists podcast.
The identification of this previously unknown bonding mechanism has opened the door for further research into disarming these bacteria and preventing them from latching onto human cells, meaning there may be an alternative to antibiotics for fighting bacterial diseases.
Find out more about the moment of discovery.
Discovering chemical harpoons
In order to live inside a host, bacteria use multiple hair-like proteins on their surface (called adhesins) to attach themselves to host cells, much like Velcro. On a molecular level, these adhesins are long, intricately arranged chains of amino acids which are able to form weak, reversible electromagnetic bonds with molecules from the host cell. The success of this attachment depends on the combined strength of many adhesins, as no single protein would be strong enough to form a stable connection between host and bacterium.
However, the research team at St Andrews has recently discovered a previously unknown host-microbe interaction. While studying Streptococcus pyogenes – a bacterium responsible for diseases such as strep throat, rheumatic heart disease, and flesh-eating disease – the team discovered an unusual feature called a thioester in an adhesin. The thioester is able to form a single strong covalent bond (sharing of electron pairs between atoms) with a molecule on a host cell, acting like a ‘chemical harpoon’ which binds rapidly and very tightly to host tissue. Whereas normal bacterial bonds can be compared to Velcro, the thioester bond is much more like superglue, creating an irreversible connection through chemical reaction.
Read more about the science behind the study in ‘An internal thioester in a pathogen surface protein mediates covalent host binding’.
Observing chemical harpoons in action
In order to understand exactly how bacteria attack human cells, the team wanted to determine the architecture of the critical proteins on their surfaces, and how these proteins interact with human cells.
The team used nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography to view a chemical harpoon protein from the strep throat bacteria Streptococcus pyogenes in atomic detail. The key discovery was made when the team went on a molecular fishing expedition with this chemical harpoon. Using mass spectrometry, a method to precisely measure the mass of molecules, they identified the blood-clotting protein fibrinogen as a binding partner. They found the bacterial adhesin was interacting with fibrinogen not by forming a weak electromagnetic bond but by a chemical reaction (forming a tight covalent bond), something not previously observed in protein interactions.
To view the interaction between bacterial chemical harpoons and human tissue, the team grew a culture of human cells and introduced an engineered variant of the bacterial adhesin. They then added a specially tailored green fluorescent protein (GFP) to the cells. GFP exhibits a bright green fluorescence when exposed to light of a particular wavelength, and was used to determine the occurrence and location of binding between the bacterial chemical harpoon protein and the host cells. The team positively determined that Streptococcus pyogenes bacteria use the chemical harpoon mechanism to bind to host cells. Depriving the adhesin of the chemical harpoon stopped the bacterial protein binding to the cells.
Find out more about observing the chemical harpoon mechanism.
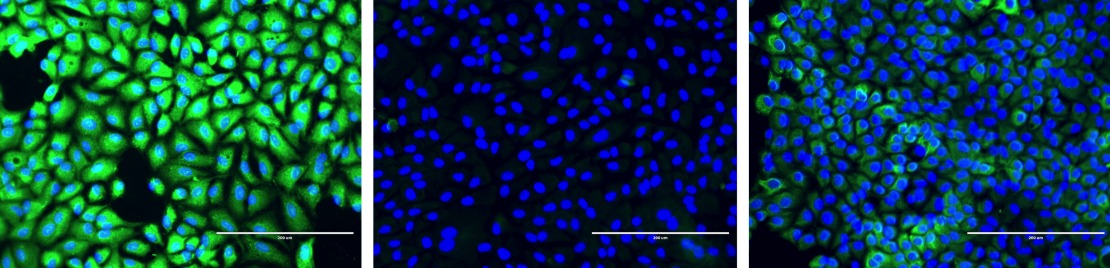
Chemical harpoon protein in action
Below are slides of thioester domains reacting with GFP proteins in human cells. In the left slide, the green fluorescence indicates that a chemical harpoon protein is bound to host cells. In the centre slide, the chemical harpoon protein is not present, and the lack of green shows that the bacterial protein is not capable of binding; all that is visible are the cell nuclei, stained in blue. In the right slide, the chemical harpoon has been disarmed using a small molecule, which acts as a prototype for preventing bacterial binding to the host.
3D model of the chemical harpoon protein
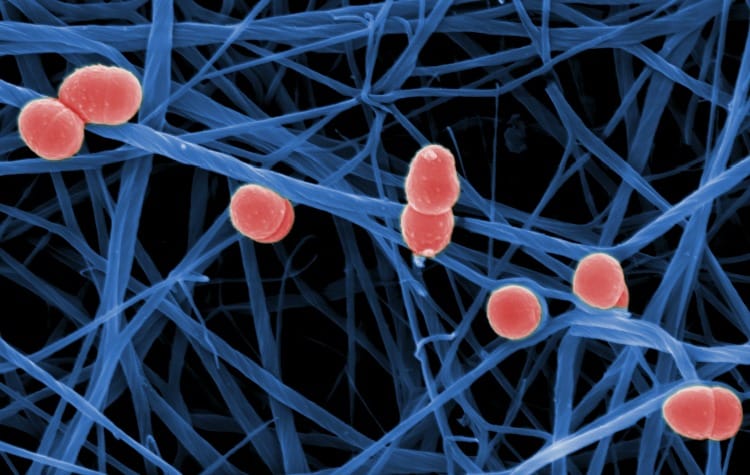
Photo created by Professor Manfred Rohde, German Helmholtz Centre for Infection Research.
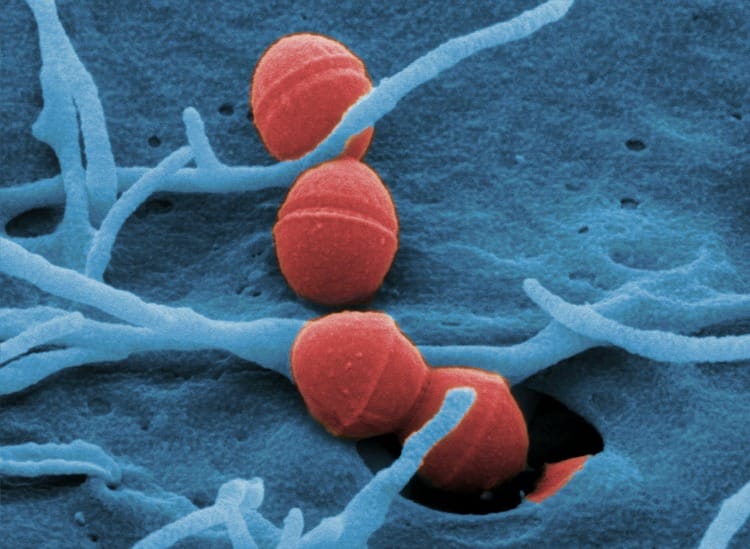
Photo created by Professor Manfred Rohde, German Helmholtz Centre for Infection Research.
The lab
Research for this project was conducted in the Biomedical Sciences Research Complex (BSRC) in St Andrews. This world-class research facility brings together scientists from biology, chemistry, medicine and physics to engage in research into the molecular basis of diseases including viral and bacterial infections.
The BSRC includes equipment and facilities in:
- protein expression and purification
- mass spectrometry and proteomics
- structural biology and biophysics (X-ray, NMR, EPR, ITC, SPR, CD)
- tissue culture
- fluorescent and confocal imaging
- bioinformatics
- synthetic chemistry
- drug discovery.
Dangerous bacteria
The discovery of the thioester chemical harpoon mechanism in Streptococcus pyogenes prompted the team to look for similar thioesters in other bacterial proteins. The team found that many, but not all, Gram-positive bacteria (bacteria which have a thick layer of a polymer known as peptidoglycan in their cell walls) have the chemical harpoon binding ability.
Warning!
Some viewers may find the following images disturbing. Viewer discretion is advised.
Deadly diseases
Gram-positive bacteria which possess chemical harpoons are responsible for causing some of the deadliest diseases in the world.
Even bacteria which do not necessarily lead to life-threatening infections can still cause discomforting diseases, such as strep throat, scarlet fever, diarrhoea and food poisoning.
Most of the images of the diseases and bacteria here are from the Centers for Disease Control and Prevention’s Public Health Image Library. The necrotising fasciitis image was taken from “Late diagnosed necrotizing fasciitis as a cause of muliorgan dysfunction syndrome: A case report” under the terms of the Creative Commons Attribution License.

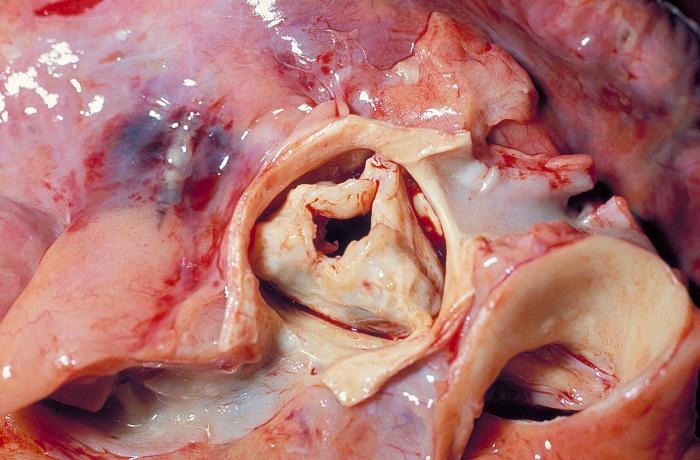
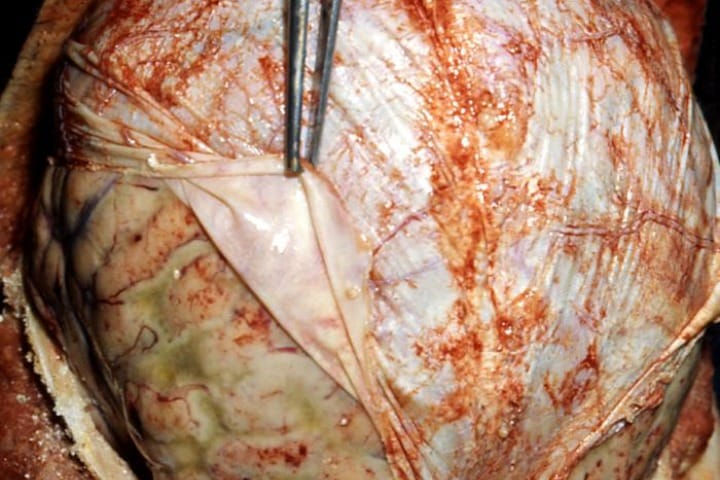
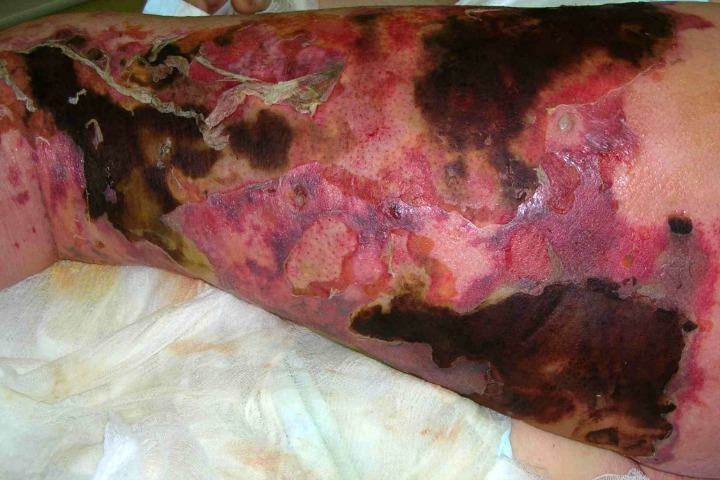
Close to home
While many bacterial diseases present unique challenges in countries with limited access to healthcare or antibiotics, some are causing problems right here in Scotland.
The MRSA superbug is a well-known antibiotic-resistant bacterium which can cause serious problems in hospitals. Although MRSA is Gram-positive, it does not appear to possess chemical harpoons. However, two PhD researchers in the team have been studying the thioester domains within two other hospital-related superbugs – VRE and Clostridium difficile – which produce several chemical harpoon adhesins.
Understanding how these two types of bacteria use the chemical harpoon to latch onto human cells could give us a better understanding of how to prevent hospital infections.
We are approaching the era where antibiotics no longer work against bacterial infections.
Miriam Weckener
VRE
Vancomycin-resistant Enterococci (VRE) are species of enterococcus bacteria which are resistant to an antibiotic known as vancomycin, as well as a number of other common antibiotics. Vancomycin has long been used as a drug of last resort to treat infections with drug-resistant bacteria. Therefore the emergence of bacteria that are resistant to this important drug is a serious concern. Enterococci commonly live in our gut and do not pose a risk to the average healthy person. However, they can cause severe infections in people who are hospitalised or seriously ill. If proper infection control measures are not taken, VRE can easily spread and persist in a hospital environment.
Types of infections which can be caused by VRE include urinary tract infections, endocarditis (inflammation of the inner layer of the heart), diverticulitis (inflammation of pouches in the large bowel wall), and meningitis. Because VRE are resistant to most common antibiotics, infections caused by VRE are extremely difficult to treat.
Ona Miller, PhD student and researcher, works with a strain of VRE called Enterococcus faecium, which is particularly a problem in the UK. She explains her work and the rising crisis of antibiotic-resistant bacteria in the video below.
C. diff
Clostridium difficile (C. diff) is a bacterium which can infect the bowel, resulting in diarrhoea as well as more serious bowel problems. C.diff is another bacterium which particularly causes problems in the hospital environment as it can very easily spread, is difficult to eradicate, and causes severe infections in patients who have been treated with antibiotics or those with weakened immune systems.
Miriam Weckener, PhD student and researcher, focuses her work on thioester adhesins in C. diff in order to understand their role in C. diff binding to human cells. She explains her work and the rising crisis of antibiotic-resistant bacteria in the video below.
Breaking bonds
With the discovery of chemical harpoons in Gram-positive bacteria, the next step involves exploring the possibility of disabling this type of bonding mechanism, which would make it impossible for bacteria to take root in the human body. Preventing bacteria from bonding with human cells in the first place could present a new way to treat bacterial infections.
In collaboration with Dr Schwarz-Linek’s research team, Dr Gordon Florence developed a prototype inhibitor which has yielded promising results in preventing thioester adhesion between bacteria and human cells.
In the first iteration, Dr Florence created a two-pronged inhibitor which was able to open and irreversibly deactivate the thioester. In a further iteration, Dr Florence added a fluorescent element to the inhibitor so that the team could use UV imaging to detect whether or not the inhibitor successfully blocked the thioester in chemical harpoon proteins.
It worked! The inhibitor prevented the bacteria from binding to human cells.
Now, Dr Florence is working on honing the prototype inhibitor. However, there have been complications in finding the correct balance between the inhibitor’s nucleophilicity and electrophilicity. The challenge that lies ahead is whether or not they will ever be able to create a viable mechanism from this prototype which could inhibit infection.
The real challenge is to both biologically and chemically validate this as a real drug target.
Dr Gordon Florence
Find out more about developing a prototype inhibitor.
Medical implications
The impact of the research has far reaching implications for global health. Streptococcal and pneumococcal infections alone cause over 1.5 million deaths per year, but diseases caused by Streptococcus pyogenes remain severely neglected. In addition, scientists and medical practitioners must be prepared for the possibility that this extremely common, but potentially devastating, pathogen could develop a resistance to antibiotics currently effective for treating infections such as strep throat.
The discovery of how Gram-positive bacteria are able to bind with human cells could potentially lead researchers to develop a form of antimicrobial intervention. The ability to prevent bacterial binding in the first place would eliminate the need to use antibiotics to treat many diseases. If bacteria are unable to gain a foothold in the human body, they won’t be able to multiply and develop into a disease.
What we’re doing could really lay the foundations for novel treatments for bacterial infections.
Ona Miller
Next steps
Discovering the chemical harpoon mechanism is only the first step in exploring alternative ways to stop bacterial infections.
We’re just scratching the start line.
Dr Gordon Florence
The next steps involve conducting more research into exactly how important the thioester proteins are for bacteria when infecting a host.
In addition, the team wants to observe how the chemical harpoon mechanism behaves in a real-life scenario by studying infections in animals rather than lab-grown human cells. This will be pursued in collaboration with research teams in the UK and Europe that have the required expertise in microbiology and animal handling.
The team will also actively pursue finessing the prototype microbial inhibitor and test whether it is a viable solution for preventing infections.
Credits
Graphics and design by the University of St Andrews digital communications team. Content written by Jennifer Hamrick, digital communications team. Video, audio and additional photography by the student-run Lightbox Creative St Andrews.
Thanks
Dr Ulrich Schwarz-Linek
Dr Gordon Florence
Ona Miller
Miriam Weckener
The Biomedical Research Sciences Complex
The Centers for Disease Control and Prevention Public Health Image Library
Alyssa Chafee and Lightbox Creative for the video and photographs.
Sources
- Baker, Edward, and Paul Young. “Host-microbe Interactions: Convergent weaponry in a biological arms race.” eLife. eLife Sciences Publications Ltd., 11 June 2015.
- "Chemical Harpoons: bacterial anchors." Interview. Audio blog post. The Naked Scientists. University of Cambridge, 24 June 2015.
- "Covalent host targeting by thioester domains of Gram-positive pathogens." Gateway to Research. Research Councils UK.
- Smuszkiewicz, Piotr, Iwona Trojanowska, and Hanna Tomczak. "Late diagnosed necrotizing fasciitis as a cause of multiorgan dysfunction syndrome: A case report." Cases Journal 1.125 (2008). BioMed Central.
- The University of St Andrews, Communications Office. “Bacteria use chemical harpoons to hold on tight to their hosts."" University of St Andrews. 2 June 2015.
- Walden, Miriam, John Edwards, Aleksandra Dziewulska, Rene Bergmann, Gerhard Saalbach, Su-Yin Kan, Ona Miller, Miriam Weckener, Rosemary Jackson, Sally Shirran, Catherine Botting, Gordon Florence, Manfred Rohde, Mark Banfield, and Ulrich Schwarz-Linek. “An internal thioester in a pathogen surface protein mediates covalent host binding.” eLife. eLife Sciences Publications Ltd., 2 June 2015.
Contact
Email: [email protected]
Phone: +44 (0)1334 46 2108