Boost for bladder cancer detection
A pioneering new method for the accurate diagnosis of bladder cancer is one step closer thanks to a £½ million funding boost.
The funding from the UK’s innovation agency, Innovate UK, will allow experts at the University of St Andrews to develop technology alongside Aberdeen-based diagnostic specialists Cytosystems.
Together they will work on the development of a cell based test coupled to software which automatically analyses digital images of the cells, resulting in an ‘extremely accurate’ test for the presence of bladder cancer.
The technology, called BladderLight™, combines novel cell collection from a patient’s urine sample and processing for biomarker expression which allows physicians to test a patient’s urine for the presence of cancer cells.
The new non-invasive kit will not only aid the initial diagnosis of bladder cancer, it will also relieve the need for traditionally uncomfortable methods of long term post treatment management of the disease such as cystoscopy and would avoid associated risks such as urinary tract infection. The patient would also be able to provide a sample at their local point of care and so forego a trip to the hospital.
Dr Peter Caie, a Senior Research Fellow in Pathology at the University’s School of Medicine, is leading the research behind the development. The technology utilises methods developed by Dr Caie that – for the first time – combine cellular shape with biomarker expression, resulting in a more accurate positive or negative result.
He said: “The cutting-edge image analysis technology involved in the BladderLight™ automated system captures complex measurements of each individual cell’s shape and size in the patient sample.
“It combines these single-cell resolution measurements with each cell’s protein biomarker expression, resulting in an extremely accurate assessment of the presence or absence of cancer cells within the urine sample.”
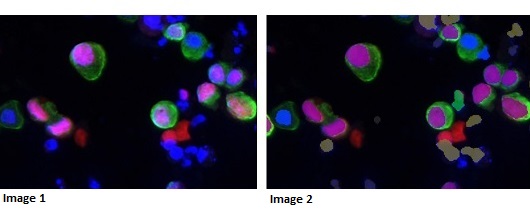
Initial results from trials of the technology have proved with 100 per cent accuracy whether a patient has cancer or not, which improves upon current clinical practice. Patients included in the trials had both low and high grade cancer – and the technology distinguished them from patients with prostate cancer, and patients with inflammation and other urinary tract disorders, which could potentially yield false positives in a more traditional test.
The funding – which follows a €3.2 million award by the EU Horizon 2020 Scheme in May – will allow Dr Caie and Cytosystems to combine their expertise in medical software development, cytopathology and urology.
Alongside the development of the technology and associated software, the latest grant will be used to secure regulatory approval for BladderLightTM in Europe and the US.
Dr Nigel McLean, Director of Product Management from Cytosystems, said: “This grant will significantly help in the development of our test for bladder cancer by reducing any further burden on already busy pathologists.
“By working with the clinical and technology experts in the University of St Andrews we can enhance the end-user experience and improve the scalability and adoption of the assay within the busy pathology environment. BladderLightTM will be of great benefit to the patient and healthcare organisations.”
Captions
Image 1: High grade cancer – biomarker (pink) expressed in urothelial cells (green)
Image 2: Image analysis mask picking up cancer cells in purple – other cell types ignored by machine learning
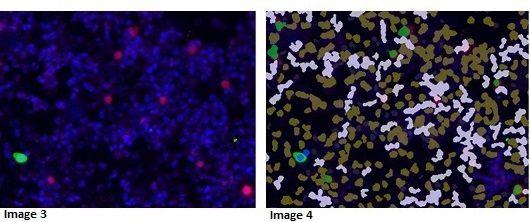
Image 3: Bladder infection (not cancer) – example of biomarker (pink) in inflammatory cells and healthy urothelial cell (green)
Image 4: Image analysis mask picking up various cells types including non-cancerous urothelial cells (blue) and biomarker positive inflammatory cells (green). No cancer cells detected. If analysis was only looking for the biomarker this patient would be flagged as a false positive.
The image analysis algorithm reports biomarker expression data co-registered to multiple shape, size and texture measurements from each individual cell. Machine learning is performed on this personalised big-data cytology report to identify the select parameters needed to identify if a patient’s sample contains bladder cancer. The machine learning identified parameters are then utilised to create a simplified clinical test applicable to routine practice.
Note to editors
Dr Peter Caie is available for interview via the press office – contacts below.
For further information on Cytosystems contact Tony Stephenson at Exitus Communications on 07899 796655 or email [email protected].
Issued by the Press Office, University of St Andrews, contactable on 01334 462530 or email [email protected].
Category Research