Key mechanism in appetite and weight control uncovered
New research from the University of St Andrews, published in Nature Communications, has uncovered a key mechanism that helps the brain regulate hunger.
Mutations in MC4R are among the most common genetic causes of severe obesity.
For some people, existing therapeutic appetite suppressants such as Wegovy or Mounjaro are ineffective or unsuitable. Yet because MC4R acts through a distinct neural circuit to regulate appetite and energy expenditure, new MC4R-targeted therapies could complement the remarkable success of these drugs.
Using state-of-the-art fluorescence microscopy and single-cell imaging, the team demonstrated that MRAP2 profoundly changes the location and behaviour of MC4R within cells. Fluorescent biosensors and confocal imaging revealed that MRAP2 is crucial to moving MC4R to the cell surface, where it can more effectively transmit signals that suppress appetite.
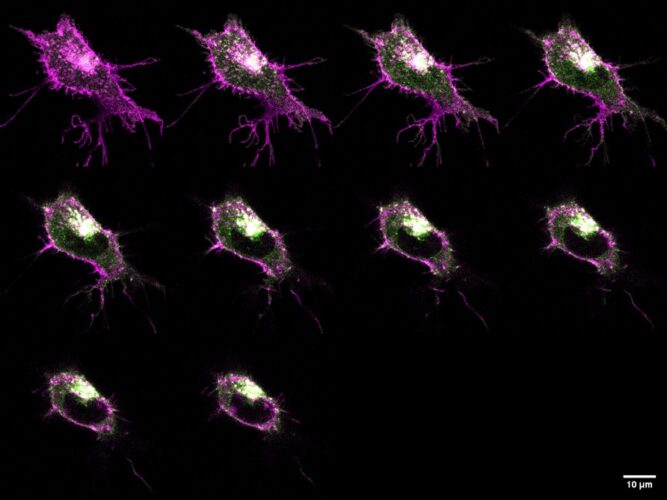
Microscopy also provided direct visual evidence that MRAP2 guides how MC4R molecules are organised. A finding confirmed through molecular brightness and fluorescence cross-correlation spectroscopy.
These results connect receptor structure with changes in signalling dynamics, highlighting MRAP2’s ability to fine-tune MC4R activity. By uncovering this new regulatory layer, the study points toward fresh therapeutic strategies that mimic or modulate MRAP2, with potential for tackling obesity and related metabolic diseases.
Within St Andrews, it featured a partnership between the schools of Medicine and Physics and Astronomy.
Co-lead author Dr Paolo Annibale, reader in the school of physics and Astronomy, said: “This work was an exciting opportunity to put to fruition in a physiologically relevant settings several of the microscopy and bioimaging approaches we have been honing over the last years to adapt to the requirements of investigating molecular processes in cells”
Co-author Dr Javier Tello, from the School of Medicine, said: “This study provided an exciting opportunity to pinpoint a fundamental mechanism underlying how the body knows when it’s full. Discoveries like this are likely to inform the development of new therapeutic strategies to combat the growing obesity epidemic.”
The research brought together expertise in live-cell fluorescence microscopy, molecular pharmacology, and structural biology across institutions in Germany, Canada, and the UK, demonstrating the power of interdisciplinary science to reveal new principles of receptor regulation.
Category University news